Drug-induced flagellate pigmentation in Adult
Alerts and Notices
Important News & Links
Synopsis

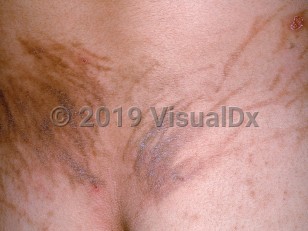
Flagellate erythema appears as linear, whip-like, erythematous streaks on the upper torso and, less frequently, on the extremities. The eruption may be preceded by severe, generalized pruritus and is itself usually very pruritic. Flagellate erythema may occur within 24 hours of receiving the culprit medication, or there may be a lag time of up to 6 months. It has a self-limited course and usually subsides, leaving a residual postinflammatory hyperpigmentation that may persist for months. In some cases, patients note minimal or absent pruritus or erythema, and hyperpigmentation is the initial presentation.
The pathogenesis of flagellate erythema is unclear. Scratching is thought to disrupt the dermal vasculature with subsequent drug leakage into the dermis. Bleomycin is metabolized by bleomycin hydrolase in the subcorneal layer. This enzyme is absent from the dermis and so the drug might accumulate there and cause its toxic effects. Immunologic mechanisms, including type I and type II responses, have also been postulated.
Pediatric Patient Considerations
Bleomycin-induced flagellate erythema and pigmentation have been reported in the pediatric population as well.
Codes
T50.995A – Adverse effect of other drugs, medicaments and biological substances, initial encounter
SNOMEDCT:
706987004 – Flagellate dermatitis
Look For
Subscription Required
Diagnostic Pearls
Subscription Required
Differential Diagnosis & Pitfalls

Subscription Required
Best Tests
Subscription Required
Management Pearls
Subscription Required
Therapy
Subscription Required
Drug Reaction Data
Subscription Required
References
Subscription Required
Last Updated:03/08/2018