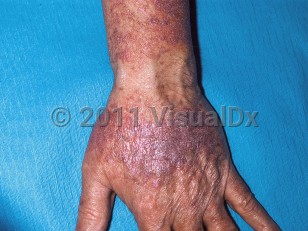
Lichenoid drug eruption in Adult
Alerts and Notices
Important News & Links
Synopsis

Multiple medications have been implicated in lichenoid drug eruptions. Classic cutaneous lichenoid drug eruptions may be caused by angiotensin-converting enzyme (ACE) inhibitors, antimalarials, beta blockers, gold, lithium, mercury amalgam, methyldopa, penicillamine, quinidine, sulfonylureas, thiazide diuretics, tumor necrosis factor (TNF)-α inhibitors, and tyrosine kinase inhibitors. Cutaneous and oral lichenoid reactions may be caused by ACE inhibitors, allopurinol, anticonvulsants, antiretrovirals, gold, ketoconazole, and NSAIDs. Photodistributed lichenoid drug eruptions may be caused by carbamazepine, chlorpromazine, diltiazem, ethambutol, quinidine, quinine, tetracyclines, and thiazide diuretics.
Typically, the eruption occurs 2-3 months after initiation of the culprit medication, although onset may be as short as a few weeks or as long as several years. Resolution may take months or up to a year after its discontinuation; however, there are reports of resolution while an individual remains on the medication. Oral lichenoid drug eruptions occur predominantly in adults, although pediatric cases have been reported.
Related topics: lichen planus, oral lichen planus, lichen planopilaris
Codes
L43.2 – Lichenoid drug reaction
SNOMEDCT:
109254000 – Lichenoid drug eruption
Look For
Subscription Required
Diagnostic Pearls
Subscription Required
Differential Diagnosis & Pitfalls

Subscription Required
Best Tests
Subscription Required
Management Pearls
Subscription Required
Therapy
Subscription Required
Drug Reaction Data
Subscription Required
References
Subscription Required
Last Updated:04/06/2021